Research
Science on “Adsorption” and “Nanospace”
We are investigating the detailed mechanism (molecular motion) of molecular processes concerning with the specific structures and properties (thermal, electrical and magnetic properties) confined in the nanometer-sized spaces, mainly from the viewpoint of the molecular motions of the adsorbed molecules. We also aim to create materials with new functions such as proton conduction, electron conduction and one-dimensional spin chains by controlling the arrangement of molecules by “space”.
Research projects
- NMR study of phase transition of nano-confined molecular assemblies induced by highly ordered nanospace
- Study on construction of one-dimensional hydrogen bonding chain using highly ordered nanospaces and its proton transport properties
- Study on freezing/melting phenomenon of molecular assembly confined in nanospaces
- Study of dynamic structures of hydrated ions confined in nanospaces
- Study on development of new methods for probing the nano-confined molecules based on NMR spectroscopy
Our latest researches
Nano-confined molecules as studied by NMR spectroscopy
– Phase change of adsorbed molecules induced by the confinement into nanospace –
– Phase change of adsorbed molecules induced by the confinement into nanospace –
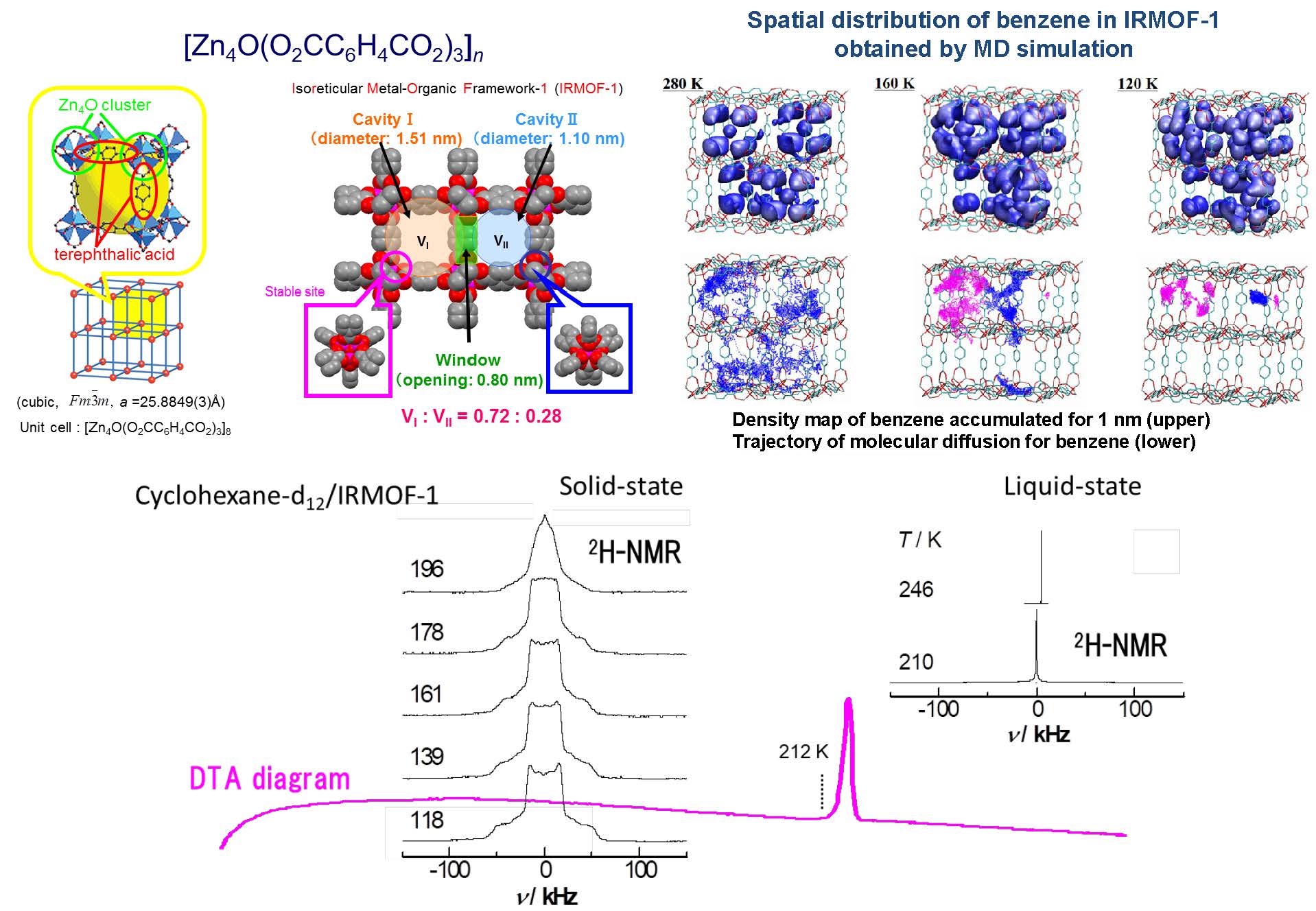
In porous coordination polymers (PCPs) and/or metal-organic frameworks (MOFs) with high crystallinity and extremely regular and periodic structure, adsorbed molecules are thought to form superlattices reflecting their pore structures. In other words, in such regular and periodic pores it is possible to form a state (adsorptive molecules) staying in the pores regularly arranged (quasi-solid) and a state freely moving around between the pores (pseudo liquid), and it is expected to show a phase change between these states. The phase change of adsorbed molecules inside such pores can also be expected to develop into temperature sensitive functional materials that can turn on physical properties depending on temperature. We discovered in the world that adsorbed molecules show phase change in porous coordination polymers called IRMOF-1 ([Zn4O(BDC)3]n; BDC = O2CC6H4CO2) .
Microdynamics of physisorption
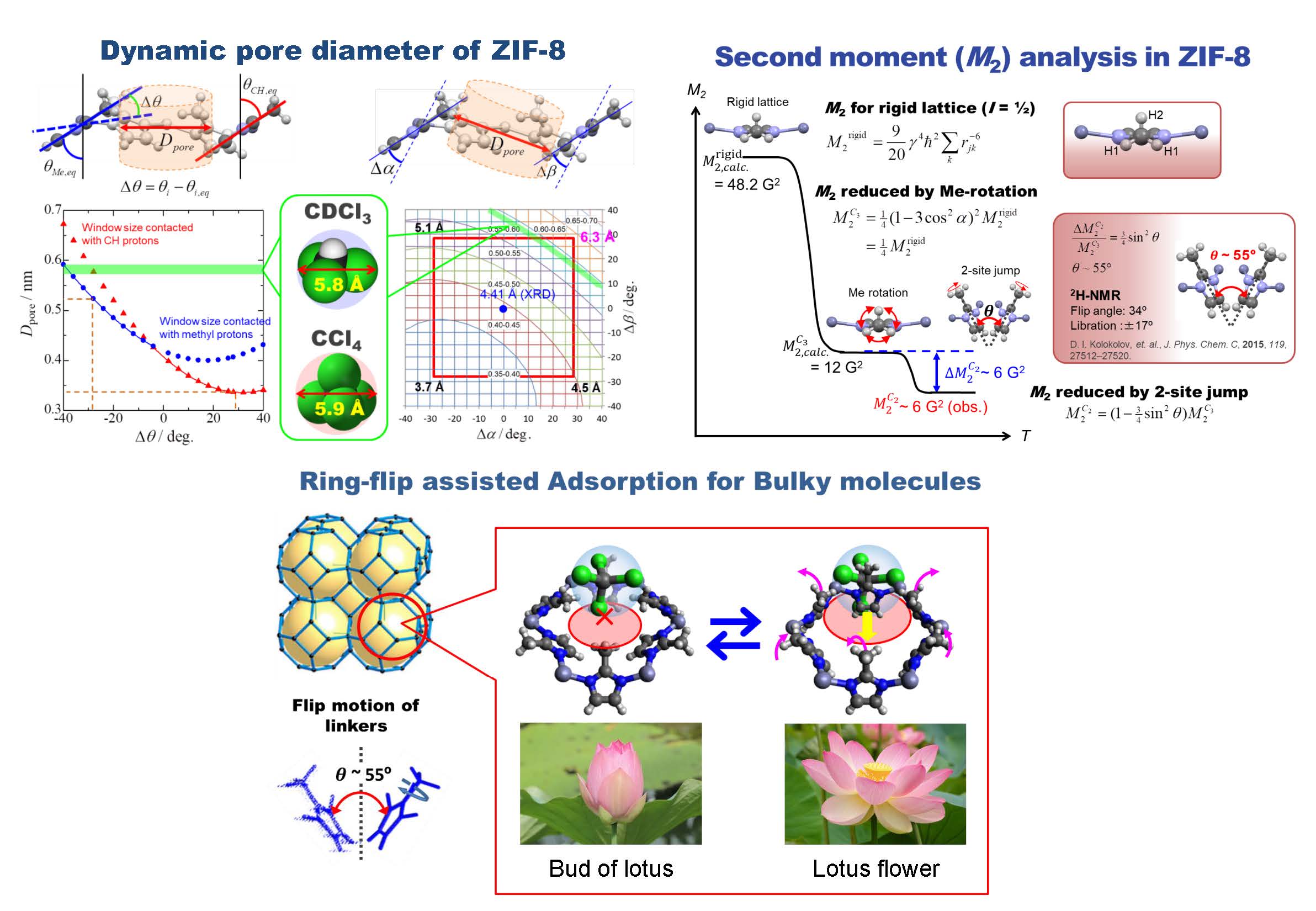
– Elucidation of adsorption mechanism of bulky molecules larger than pore size –
A type of porous coordination polymers, called “Zeolitic Imidazolate Frameworks-8” is constructed from Zinc(II) ions and 2-methylimidazolate anion of (MeIm-) as a linker (ZIF-8) [Zn(MeIm)2]n. Their crystal has a sodalite structure where the micropores of 11.6 Å diameter are connected three-dimensionally with eight 6-membered ring apertures (diameter 3.4 Å) each other. One of the most interesting phenomena is that this material can adsorb bulky molecules larger than the opening diameter of 3.4 Å, which is determined by X-ray diffraction. This unique phenomenon is considered to concern closely with the flexibility of the linker moieties of ZIF-8. We revealed that the large amplitude flip motion of the 2-methylimidazolate ring expand pore size transiently, and makes it possible to pass the bulky molecules through the apertures. This mechanism seems to be able to explain the adsorption of bulky molecules into many porous coordination polymers as a new adsorption mechanism, which has not found in ordinary porous materials having a rigid framework structure such as zeolite and activated carbon so far.